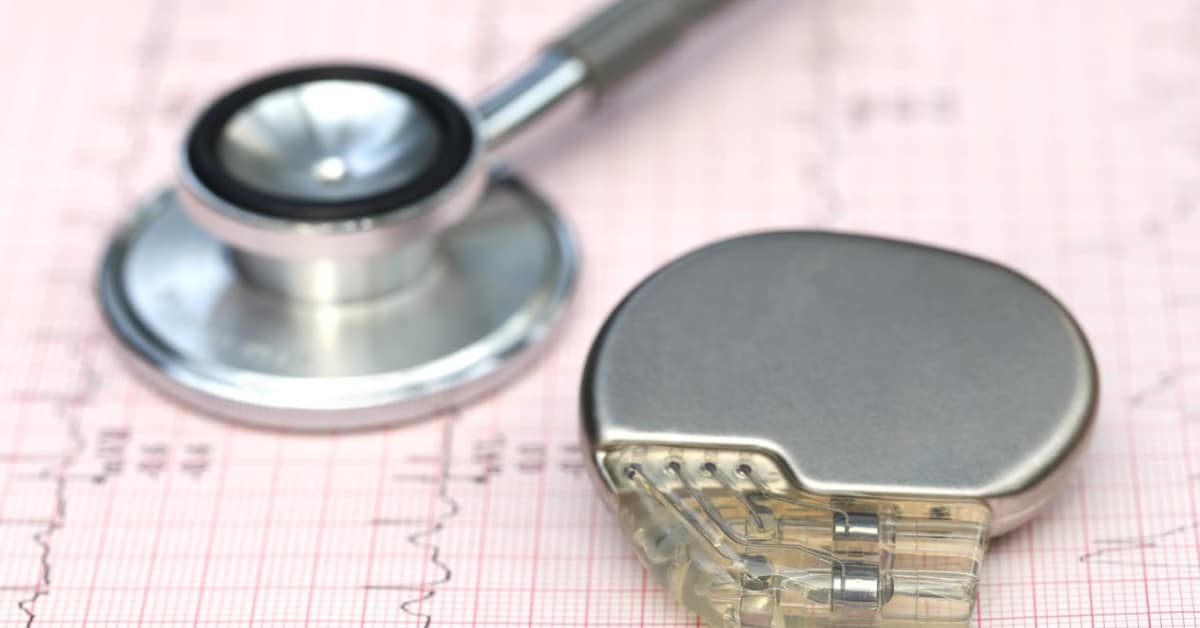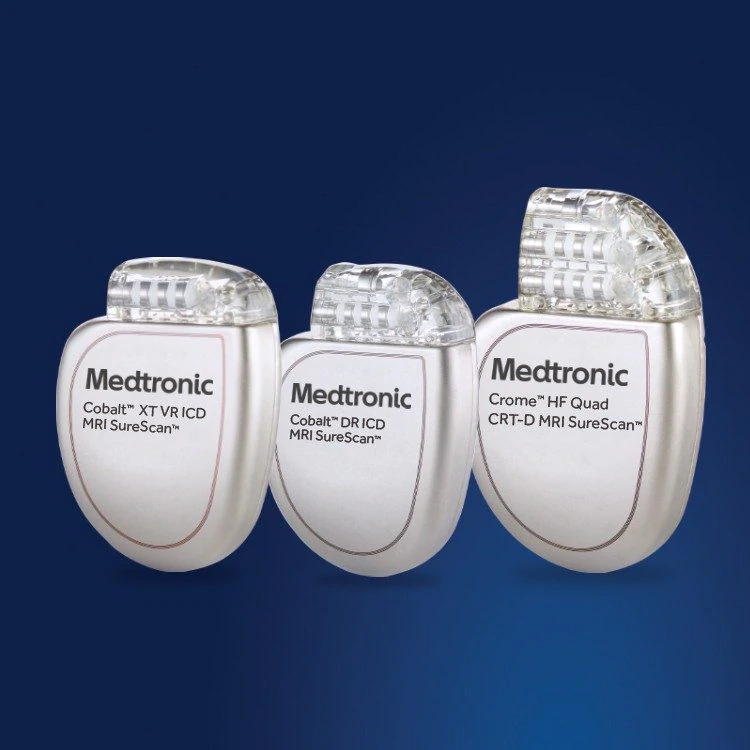
FDA announces recall of nearly 88,000 implantable cardiac devices due to risk of serious injury or death
Perioperative Interrogation of Medtronic Cardiovascular Implantable Electronic Devices: A Guide for Anesthesiologists

PDF) Design of the Pacemaker REmote Follow-up Evaluation and Review (PREFER) trial to assess the clinical value of the remote pacemaker interrogation in the management of pacemaker patients