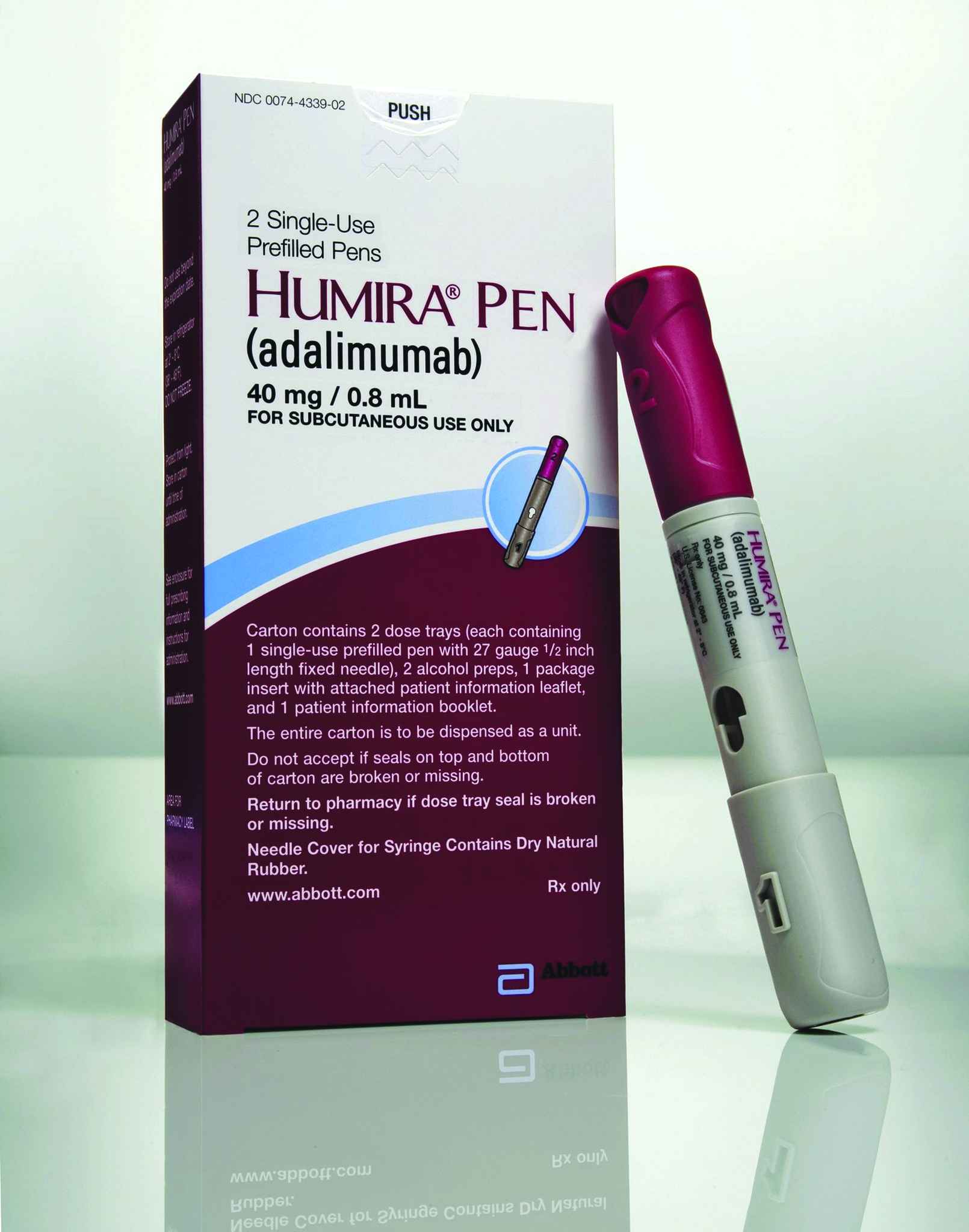
Humira Pen Psoriasis Starter Pack Subcutaneous Reviews and User Ratings: Effectiveness, Ease of Use, and Satisfaction

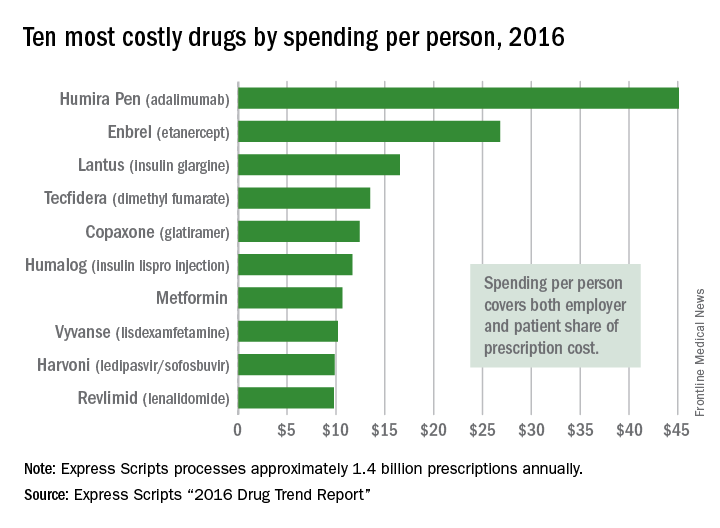
Humira rings up $20.7B in 2021, but AbbVie still mum on post-biosimilar expectations | Fierce Pharma
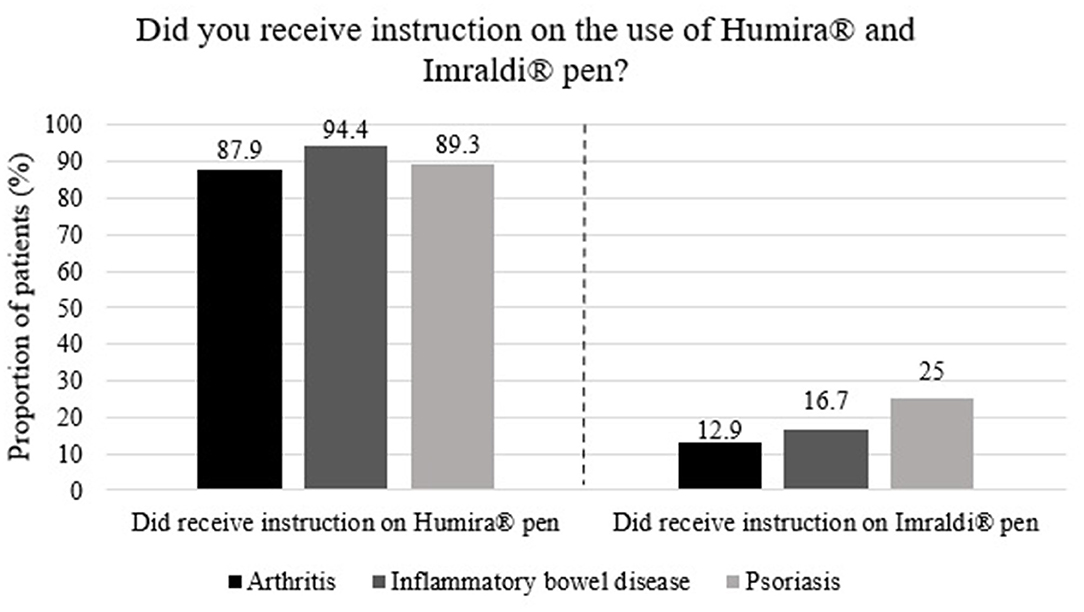
Frontiers | A Patients' Perspective Towards the Injection Devices for Humira® and Imraldi® in a Nationwide Switching Program

PDF) HUMIRA (R) Pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab

Humira Crohn's Disease Starter Pack Disease-Modifying Antirheumatic Agent Adalimumab, Preservative Free 40 mg / 0.8 mL Subcutaneous Injection Prefilled Auto-Injector Pen 6 Doses - Suprememed
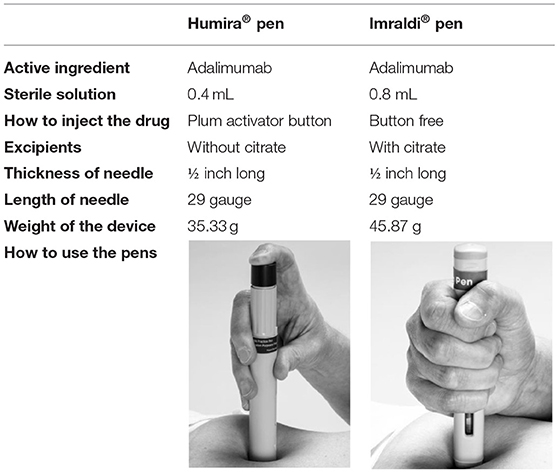







:quality(70)/arc-anglerfish-arc2-prod-tronc.s3.amazonaws.com/public/NFS64DMH4RCY5MWFEUFUW4IBY4.jpg)